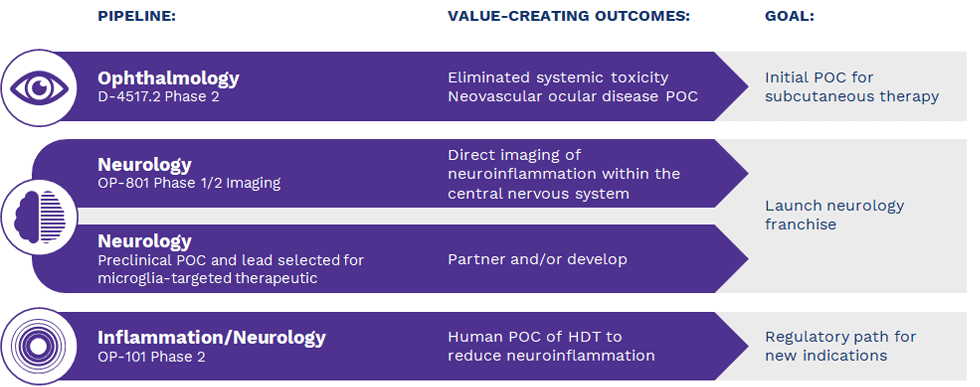
Ashvattha Therapeutics Announces First Patient Enrolled in Expanded Phase 1/2 Study of Imaging Agent 18F-OP-801 in Additional Neurological Indications
The study will evaluate the safety, tolerability, and ability of 18F-OP-801 to cross the blood-brain barrier and selectively target neuroinflammation in individuals with amyotrophic lateral