Ophthalmology Pipeline Represents a Paradigm Shift in Treatment
THE NEED
- Over 10M eye injections per year = Heavy treatment burden on patients and doctors
- Approved and prospective drugs require a retinal ophthalmologist, a caregiver to drive to/from visits, and an expensive treatment
OUR SOLUTION
- Patient administers drug at home with autoinjector subcutaneously up to once per month (potential for oral administration)
- Market Research = > $3.5 B peak US sales
51 US retinal ophthalmologists, 5 payers
Discount to Eylea, rebates, market access (conservative)
Patient market research ongoing
- Lower cost – manufactured at up to 1/10 the cost of other approaches
HD Targets Ocular Neovascularization After Systemic Dose
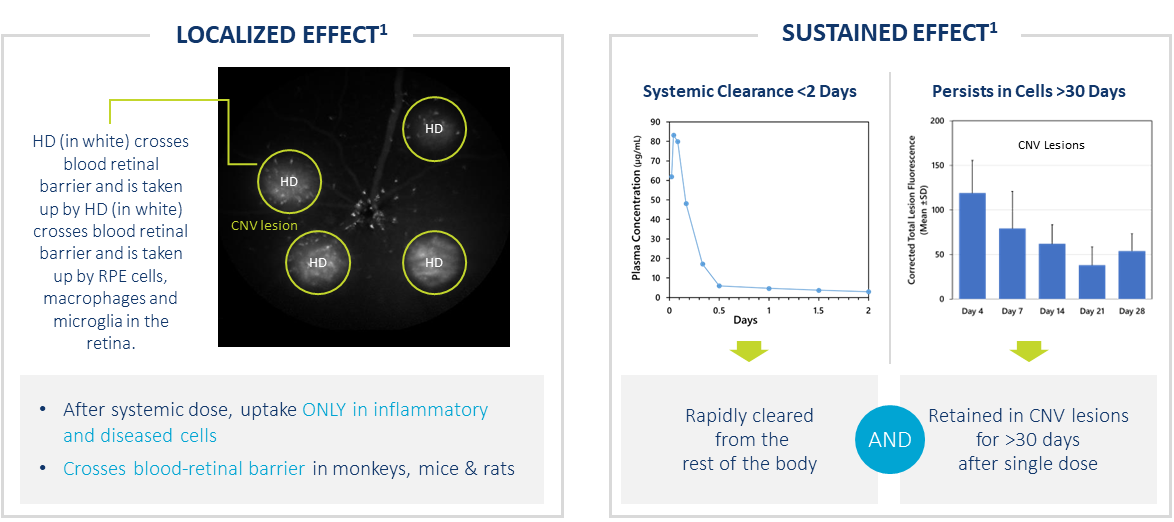
- After systemic dose, uptake ONLY in activated cells in regions of inflammation
- Crosses blood retinal barrier in monkeys, mice & rats
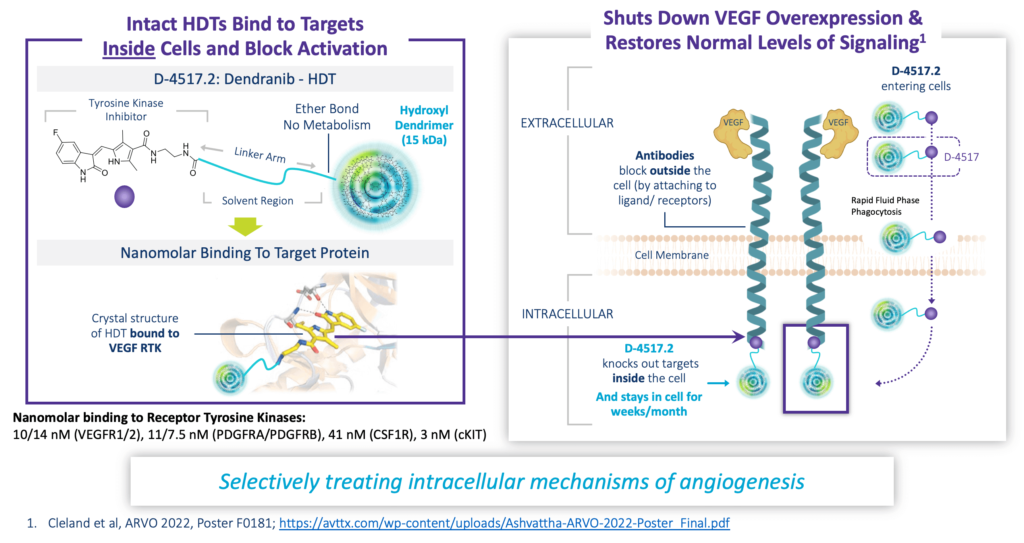
D-4517.2: First-in-Class Home Administered Wet AMD Therapy
Mouse Laser CNV Model: Single Subcutaneous or Oral D-4517.2 Dose on Day 1
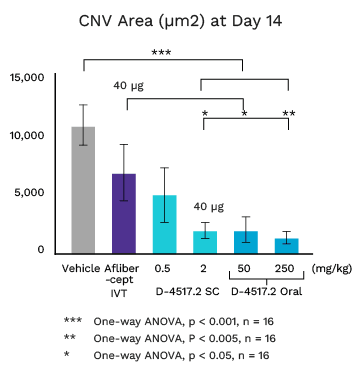
Once Every 2+ Weeks or Oral
D-4517.2 at 2 mg/kg (40 µg) subcutaneous (SC) dose equivalent to Eylea (aflibercept) intravitreal (IVT) dose (40 µg) at Day 14
Oral D-4517.2 doses equally or more effective
Human doses to achieve equivalent effect are safe and well tolerated
Our Approach
- Patient administers drug at home with autoinjector up to once per month
(potential for oral administration)
Market Expansion
- Patients in rural areas receive convenient treatment (especially parts of China and India)
- Diabetic patients maintain compliance (treated for decades)
- Treatment of earlier stage of AMD
- Lower cost – manufactured at up to 1/10 the cost of other approaches
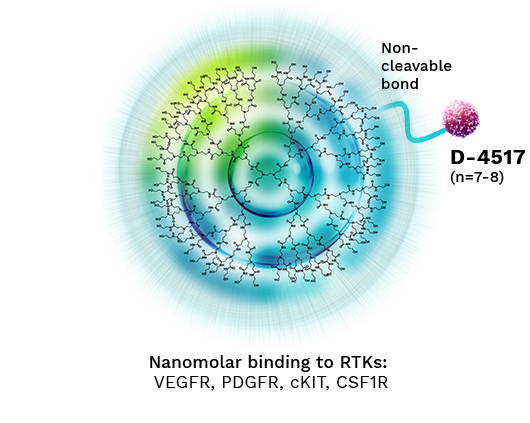
D-4517 Alters Pharmacology through Non-Cleavable Dendrimer
| Sunitinib (Sutent™) | D-4517 |
| Liver toxicity (decreased size/increased ALT/AST) | No observed effect |
| Proteinuria, decrease in kidney size (males) | No observed effect |
| Hypoglycemia (females) | No observed effect |
| Decrease in heart size (males) | No observed effect |
Same sunitinib exposure in both groups
D-4517
proprietary Sunitinib analog
D-4517.2: Safety Established in Healthy Humans
Phase 1, Single Dose – SC Doses Up to 1 mg/kg
Study Objectives:
Complete safety, PK, metabolism, clearance and safety of single SC dose administration in adult subjects
Study Results Summary:
- Safe and well tolerated with only mild transient injection site reactions in a few patients (technique related)
- No changes in safety labs
- PK translates to effective doses in preclinical models
D-4517.2: Phase 2 Proof of Concept in Wet AMD and DME Patients
Single Dose Study
Study Objectives:
Safety and pharmacodynamic activity (based on fluid level and visual acuity) of single SC dose administration in adult subjects with wAMD and DME
Study Results Summary:
- Safe and well tolerated
- No changes in safety labs
- Fluid level and visual acuity measurements suggest that SC D-4517.2 localizes to and is active in the eye
- Results support initiation of treat-to-maintain study paradigm with chronic dosing
Chronic Dose Study
Study Objectives:
The primary objectives of the study are to evaluate the safety and tolerability of multiple D-4517.2 subcutaneous doses and the ability of different D-4517.2 dose regimens to maintain fluid level and visual acuity following a single intravitreal anti-VEGF treatment. An important metric for assessing the clinical benefit of D-4517.2 in this treat-to-maintain paradigm is the time to anti-VEGF rescue following the initial dose.
Study Results Expected in First Half of 2024
TIMELINES
Stage 1, Phase 2 LPI in 1H 2023
- Stage 1, Phase 2 data in 2H 2023
Stage 2, Phase 2 FPI in 2024
Launch wet AMD Stage 2 and Separate DME Phase 2 in Parallel
Projected Product Launch = 2028
D-4517.2 Commercial Assessment

