Migaldendranib (MGB)
Migaldendranib (MGB) is a potent anti-angiogenic nanomedicine (“dendranib”) that crosses the blood-retinal barrier and selectively targets activated microglia, macrophages and retinal pigment epithelial cells in the eye. MGB has the potential to change the current treatment paradigm for neovascular age-related macular degeneration (wet AMD) and diabetic macular edema (DME) by offering an at-home dosing option by a subcutaneous route of administration rather than delivery via intravitreal injection (injection into the eye). MGB is in a Phase 2 clinical trial in patients with wet AMD or DME.
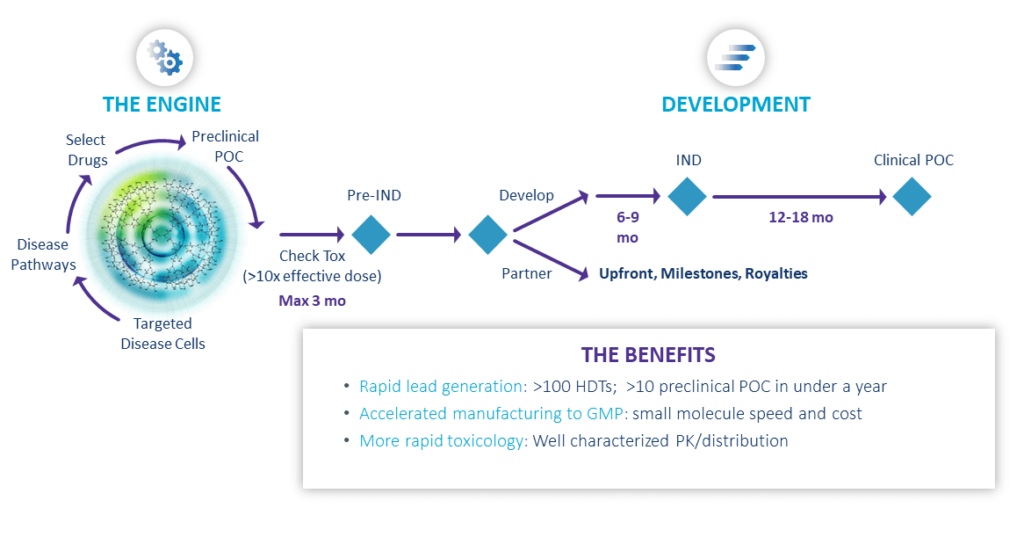
Engine Rapidly Delivers Breakthrough Therapies
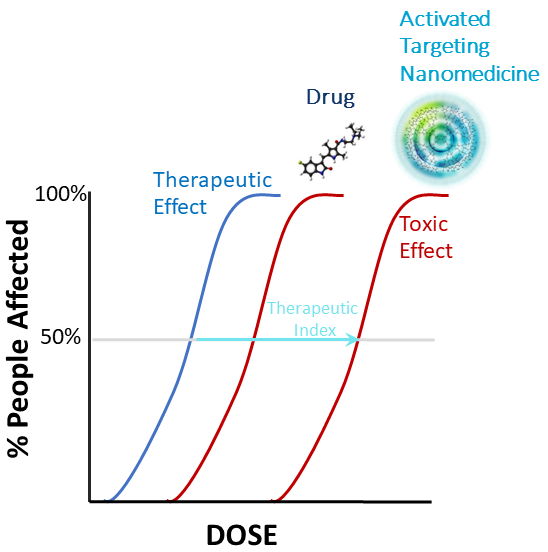
Opening the Therapeutic Index
- Uptake only in activated cells in regions of inflammation
- No detectable metabolism and drug clearance mediated by the kidney
- Rapid clearance (<1-2 days in humans) with retention in cells for up to a month (less systemic exposure)
- Human demonstration of HDT with drug that is normally toxic
- Example
- MGB, potent anti-angiogenic agent, no observed adverse effects in humans at the therapeutically effective dose
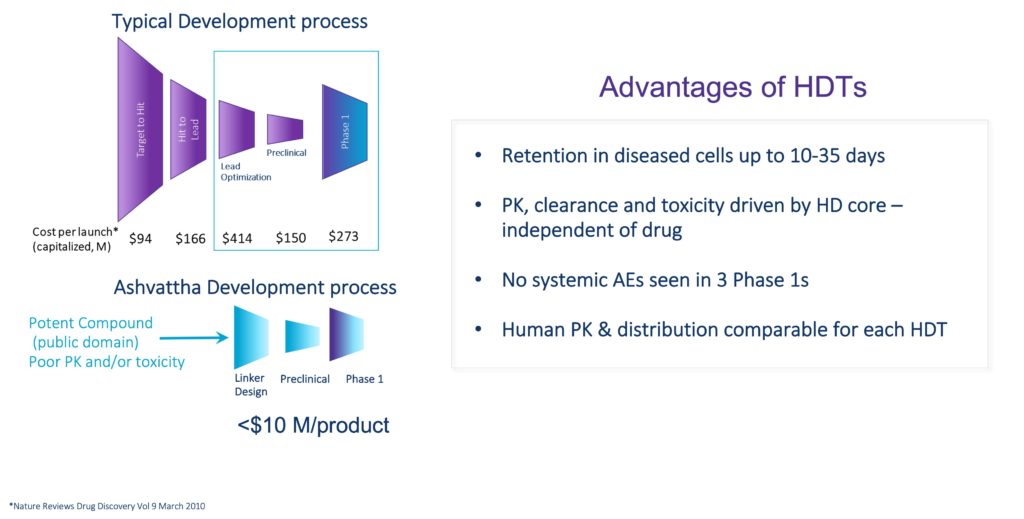
Disrupting the Drug Development Paradigm