Potent Anti-Inflammatory: OP-101
THE NEED
Current anti-inflammatory drugs cause broad immune suppression leading to side effects and secondary infections
OUR SOLUTION
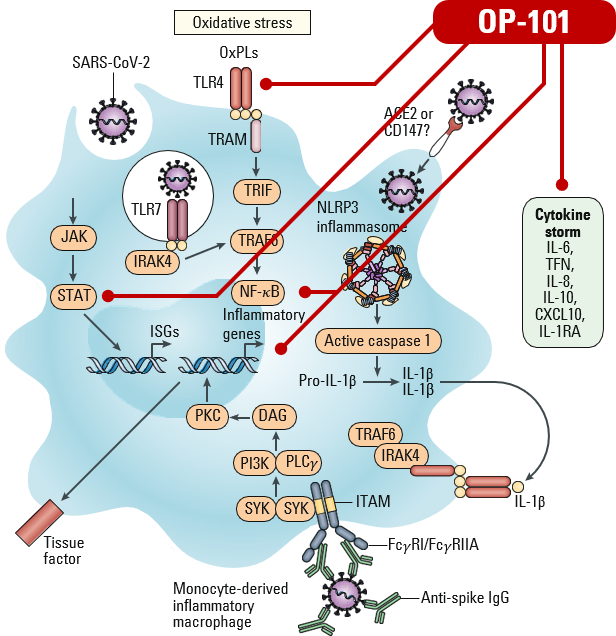
Targets the cause of hyperinflammation at its source, including activated macrophages attacking lungs and organs
Restores the number of macrophages to normal levels, preventing further inflammation from occurring
Profound effects of reducing neuroinflammation in multiple animal models
Converts activated proinflammatory macrophages and microglia to normal anti-inflammatory phenotype, preventing further inflammation from occurring
OP-101: A Targeted Selective Anti-Inflammatory Agent
Dexamethasone does appear to be life saving for those sickest patients …but it's a pretty big sledgehammer in terms of what it does to the immune system. Maybe there's something a little more subtle that would be even more effective with less in the way of side-effects.
− Dr. Francis Collins, Director of the NIH
Merad & Martin Nature Rev 2020
Mechanism of Action
- OP-101 (acetylcysteine zidrimer) is selectively taken up by activated macrophages – More potent than steroids across multiple models
- Mechanism demonstrated in animal models of lipopolysaccharide (LPS) induced inflammation (ARDS, TB, neuroinflammation, etc.)
- After uptake, OP-101 returns macrophage to “normal” shutting down multiple pathways resulting in inflammation.
- OP-101 also reduces oxidative stress, a process that can damage cells (corticosteroids do not address oxidative stress)
Kannan 2012; Nance 2017
OP-101: Safety Established in Healthy Humans
2x Phase 1, Single-dose – Intravenous Doses Up to 40 mg/kg
Subcutaneous Doses Up to 8 mg/kg
Excellent Safety Profile to Date
5-10x Therapeutic Window – Predicted Therapeutic Dose = 2-8 mg/kg
Study Objectives:
Complete safety, Pharmacokinetics (PK) metabolism, clearance and safety of single-dose administration in adult subjects
Study Results Summary:
- All doses well tolerated with no clinical adverse events
- No metabolites detected in urine
- Complete recovery of the drug in the urine in healthy humans (no cellular uptake)
Excellent Safety Profile to Date
5-10x Therapeutic Window – Predicted Therapeutic Dose = 2-8 mg/kg
Phase 2a Trial in Severe COVID-19 Patients
Double Blind, Placebo Controlled Study of OP-101 in Severe COVID-19 Patients
OP-101 Dosed at 2, 4 or 8 mg/kg IV
Top-line Study Results
- OP-101 improved survival compared to standard of care placebo control (82.4% vs 42.8%)
- Significant and sustained reduction in pro-inflammatory biomarkers above corticosteroids alone (p < 0.01)
- Significantly reduced neuronal injury biomarker (p < 0.001) to healthy control levels
- Well tolerated with no drug related adverse events
- Shuts down multiple pathways associated with hyperinflammation and crosses the blood-brain barrier
Study Published: Gusdon et al., Sci. Transl. Med. 14, eabo2652 (2022) 20 July 2022
