Neuroinflammation Pipeline Enables Patient Selection
THE NEED
Chronic neuroinflammation, inflammation in the brain or spinal cord, correlates with poor outcomes in a wide range of diseases (e.g. ALS, MS, Alzheimer’s, Parkinson’s)
Neuroinflammation is difficult to diagnose, and current diagnostics lack sufficient selectivity
OUR SOLUTION
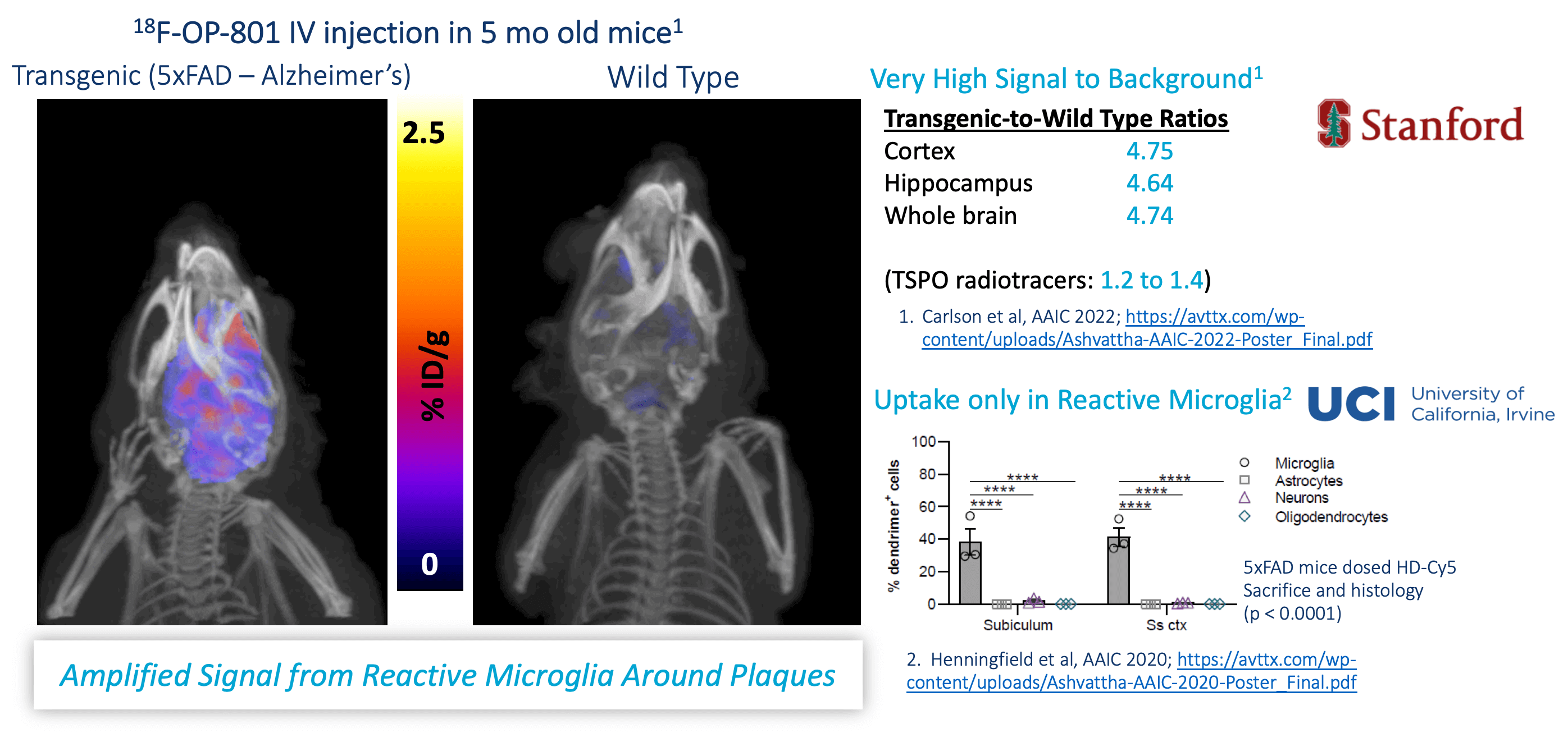
- Developed a biomarker (18F-OP-801) that is selectively taken up by activated microglia in regions of neuroinflammation in multiple animal models
- Provides a method to measure nanomedicine uptake in the brain to select patients
- Because all HDs distribute the same way in the body, the amount of 18F-OP-801 in the brain provides an estimate of the amount of nanomedicine that will get to the same cells in the brain
- Our nanomedicines have been observed to cross BBB in 7 animal species only when neuroinflammation is present
Leveraging OP-801 to Achieve Human POC
Ability to Ensure Nanomedicines Target Diseased Brain Tissues
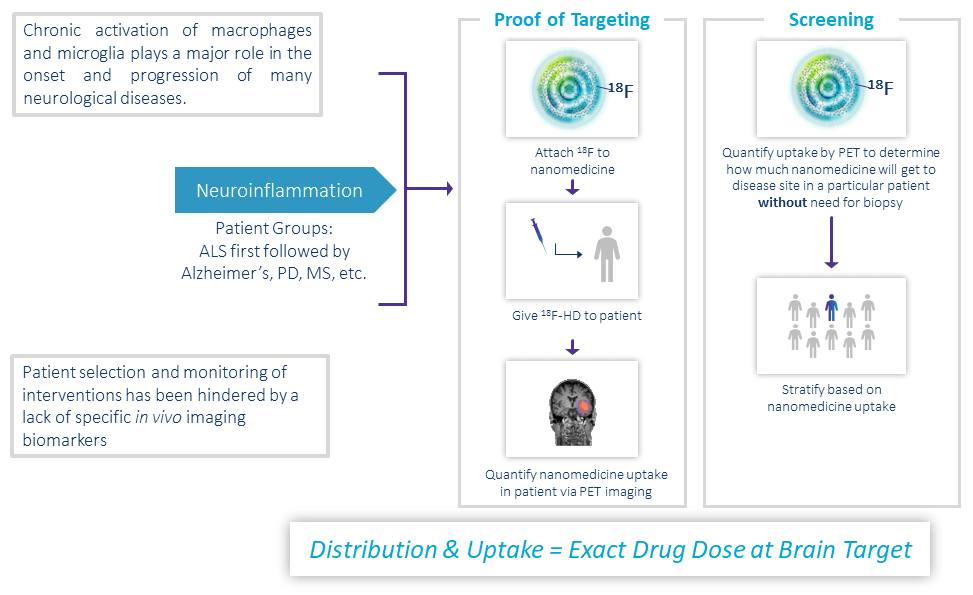
18F-OP-801 (18F-HD) Increases Trial Success: Patient Selection, HD Target Engagement
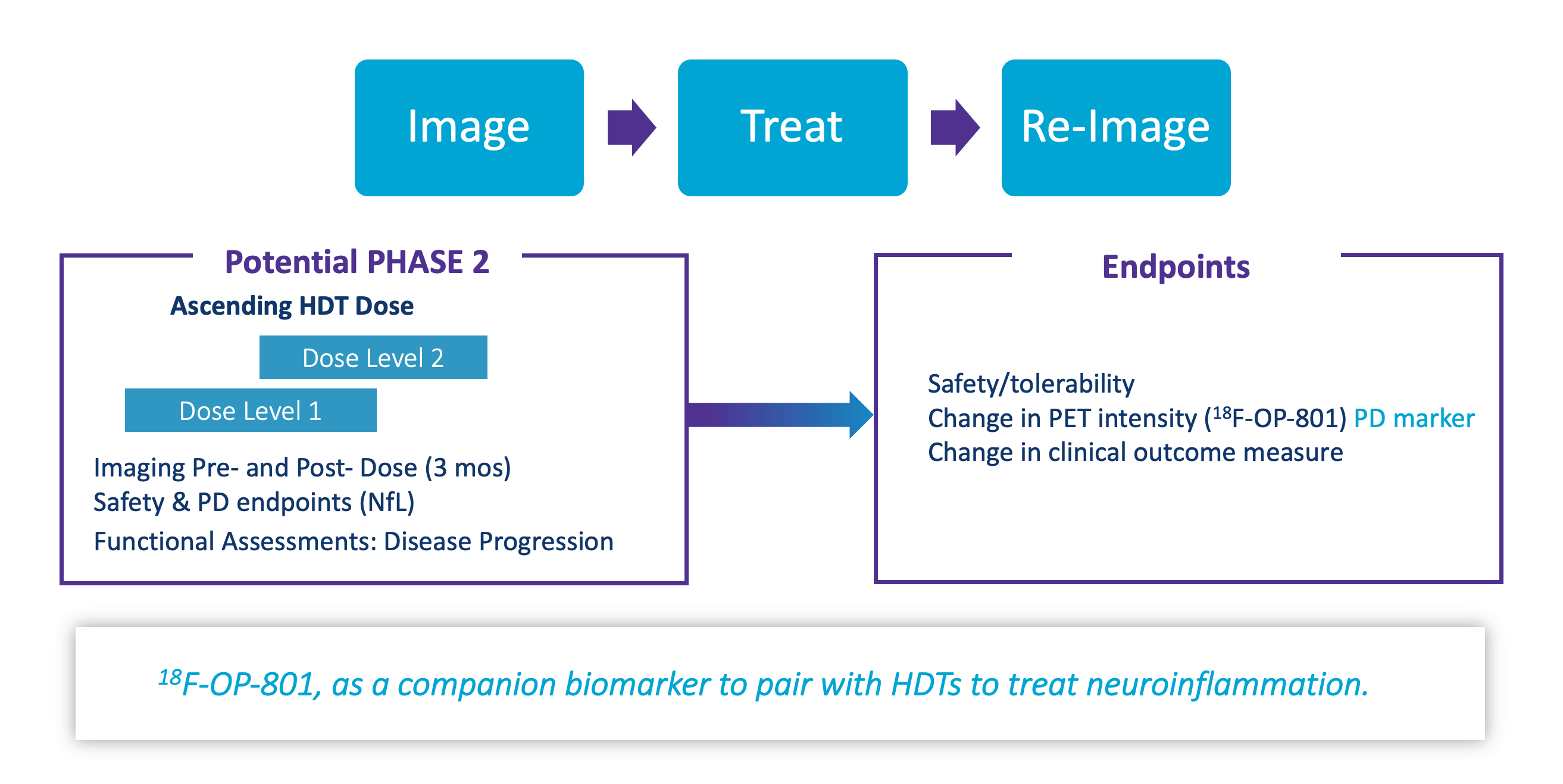
Selective Targeting to Neuroinflammation in Alzheimer’s Disease Mice
OP-801: Phase 1/2 Proof of Targeting Neuroinflammation in ALS Patients
Phase 1/2 – Age Matched Healthy Volunteers and ALS Patients
- Safety & Tolerability of 18F-OP-801 (IV dose)
- Pharmacokinetics & Dosimetry of 18F-OP-801
- Optimize PET scan for future studies – Optimal Dose & Scan Time
- ALS vs Normal Healthy Adults PET images – Demonstration of Selectivity
(analogous to 5xFAD mouse data)
TIMELINES
- LPI in 2H 2023
Expand to Image-Treat-ReImage Study in 2024
