Ophthalmology Pipeline Represents a Paradigm Shift in Treatment
THE NEED
- Over 10M eye injections per year = Heavy treatment burden on patients and doctors
- Approved and prospective drugs require a retinal ophthalmologist, a caregiver to drive to/from visits, and an expensive treatment
OUR SOLUTION
- Patient administers drug at home with autoinjector subcutaneously up to once per month (potential for oral administration)
- Market Research = > $3.5 B peak US sales
51 US retinal ophthalmologists, 5 payers
Discount to Eylea, rebates, market access (conservative)
Patient market research ongoing
- Lower cost – manufactured at up to 1/10 the cost of other approaches
HD Targets Ocular Neovascularization After Systemic Dose
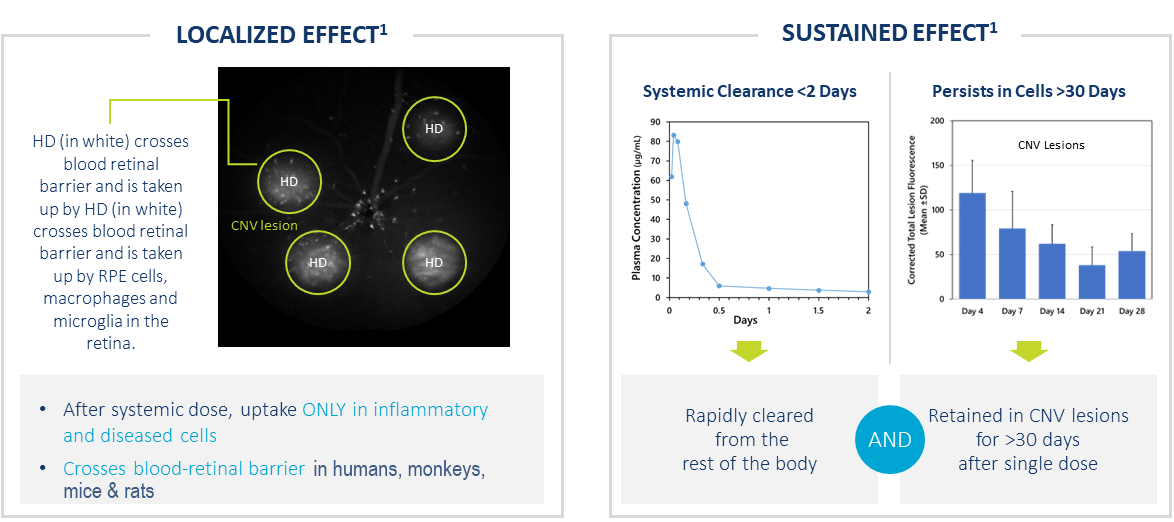
- After systemic dose, uptake ONLY in activated cells in regions of inflammation
- Crosses blood retinal barrier in humans, monkeys, mice & rats
MGB: Effective as Single Subcutaneous (SC) Dose1
Single SUBCUTANEOUS (SC) MGB Dose on Day 1
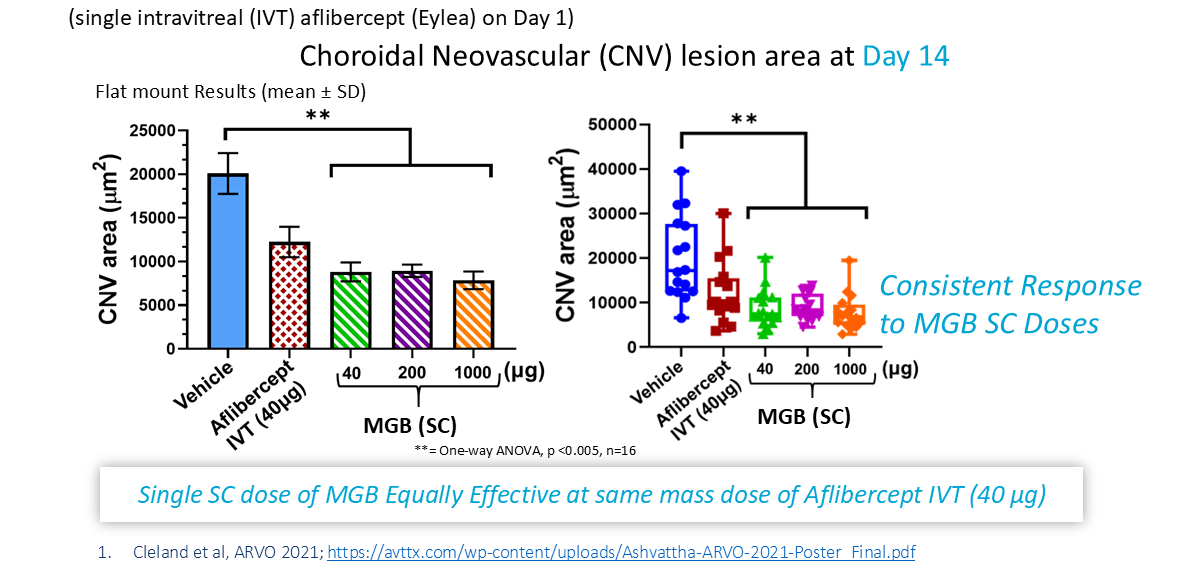
Once Every 2+ Weeks
MGB at 2 mg/kg (40 µg) subcutaneous (SC) dose equivalent to Eylea (aflibercept) intravitreal (IVT) dose (40 µg) at Day 14
Human doses to achieve equivalent effect are safe and well tolerated
Our Approach
- Patient administers drug at home with autoinjector up to once per month
(potential for oral administration)
Market Expansion
- Patients in rural areas receive convenient treatment (especially parts of China and India)
- Diabetic patients maintain compliance (treated for decades)
- Treatment of earlier stage of AMD
- Lower cost – manufactured at up to 1/10 the cost of other approaches
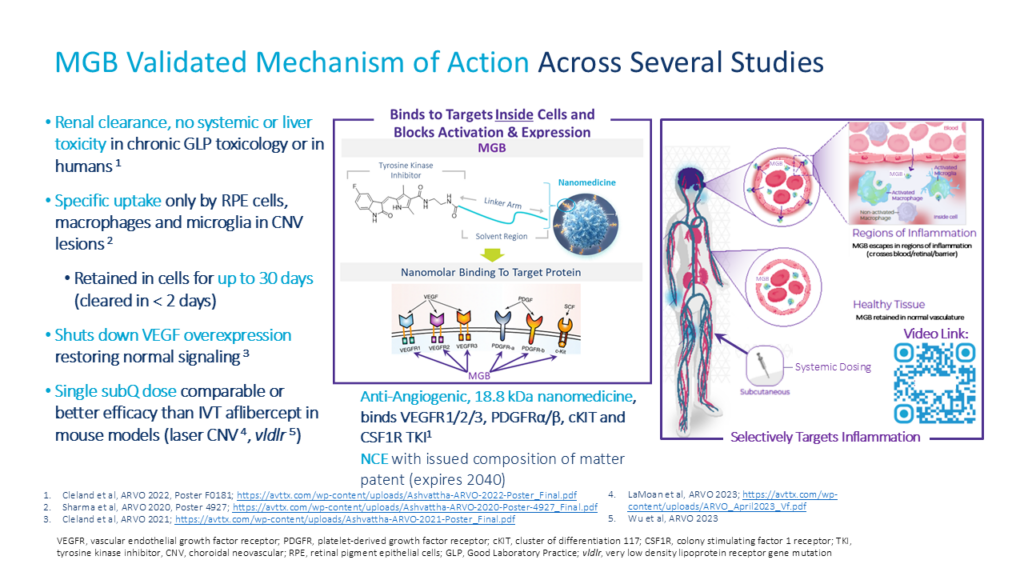
D-4517 Alters Pharmacology through Non-Cleavable Dendrimer
| Sunitinib (Sutent™) | D-4517 |
| Liver toxicity (decreased size/increased ALT/AST) | No observed effect |
| Proteinuria, decrease in kidney size (males) | No observed effect |
| Hypoglycemia (females) | No observed effect |
| Decrease in heart size (males) | No observed effect |
Same sunitinib exposure in both groups
D-4517
proprietary Sunitinib analog
MGB: Safety Established in Healthy Humans
Phase 1, Single Dose – SC Doses Up to 1.5 mg/kg
Study Objectives:
Complete safety, PK, metabolism, clearance and safety of single SC dose administration in adult subjects
Study Results Summary:
- Safe and well tolerated with only mild transient injection site reactions in a few patients (technique related)
- No changes in safety labs
- PK translates to effective doses in preclinical models
MGB: Phase 2 Proof of Concept in Wet AMD and DME Patients
Single Dose Study
Study Objectives:
Safety and pharmacodynamic activity (based on fluid level and visual acuity) of single SC dose administration in adult subjects with wAMD and DME
Study Results Summary:
- Safe and well tolerated
- No changes in safety labs
- Fluid level and visual acuity measurements suggest that SC MGB localizes to and is active in the eye
- Results support initiation of treat-to-maintain study paradigm with chronic dosing
Chronic Dose Study
Study Objectives:
The primary objectives of the study are to evaluate the safety and tolerability of multiple MGB subcutaneous doses and the ability of different MGB dose regimens to maintain fluid level and visual acuity following a single intravitreal anti-VEGF treatment. An important metric for assessing the clinical benefit of MGB in this treat-to-maintain paradigm is the time to anti-VEGF rescue following the initial dose.
Study Results Expected in First Half of 2025
TIMELINES
Stage 1, Phase 2 LPI in 1H 2023
- Stage 1, Phase 2 data in 2H 2023
Stage 2, Phase 2 FPI in 2024
Launch wet AMD Stage 2 and Separate DME Phase 2 in Parallel
Projected Product Launch = 2028
MGB Commercial Assessment

